
In This Issue:
- Diamondback moth, Japanese beetle, Asiatic garden beetle, Colorado potato beetle updates and management
- Disease forecasting updates for potato early blight and late blight
- Cucurbit downy mildew updates
- Blackleg and aerial stem rot in potato – updates and management
Vegetable Insect Update – Russell L. Groves, Professor and Department Chairperson, UW- Madison, Department of Entomology, 608-262-3229 (office), (608) 698-2434 (cell), e-mail: rgroves@wisc.edu
Vegetable Entomology Webpage: https://vegento.russell.wisc.edu/
Diamondback moth. (https://vegento.russell.wisc.edu/pests/caterpillar-pests- of-cole-crops/)
A full generation of Diamondback moth (DBM: Plutella xylostella) has just been completed in southern Wisconsin. Continue to scout fields weekly throughout the remainder of the season for early season damage. Check plants carefully, even if no feeding damage is apparent, and examine the underside of leaves for small larvae and eggs that will hatch into small caterpillars in several days. Examine the lower leaves of the plant for the larvae of DBM and especially look for the characteristic ‘window-paning’ damage. Caterpillars cause varying amounts of damage depending on the plant’s maturity, and the stage of their development. Early stages of DBM infestation can be initially be quite minor, but later stages of development and unchecked populations can reach severe levels of infestation rapidly.
Late-stage caterpillars of DBM are nearing completion of the 1st full generation at the Arlington and Agricultural Research Station. They have completed their larval stages as we are observing the woven pupae on the undersurface of leaves now. While scouting, you will often observe the feeding damage associated with larval DBM feeding and immediately adjacent will be the pupa within a silken cocoon.
Keep a record of which stage of development is present and the percentage of plants infested. This information will be useful for monitoring whether the population is increasing or decreasing. Diamondback moth eggs are tiny, flat, circular and cream-colored, laid singly or in small clusters on the leaves. The larvae are small (up to 3/8 inches long at maturity) pointed at both ends and range in color from green to yellow. The diamondback larvae are commonly
found on the leaf surface and will wiggle back and forth when disturbed, often falling from the plant. Adults are small grayish- brown, night-flying moths with a 1-inch wingspan. Diamond- shaped markings on the wings are evident when the wings are folded over the back at rest. Behavioral differences between the caterpillars can aid in their identification. The cabbageworm will usually remain motionless when disturbed, whereas DBM will wiggle its body around.
Treatment thresholds are well established and based on the percent of infestation by any lepidopteran species. Economic thresholds (ETs) vary based on the stage of crop development. Cabbage, broccoli and cauliflower in the seedbed are particularly susceptible to damage. Therefore, control measures are warranted when 10% of the plants are affected. Between transplant and cupping, the ET is raised to 30%, from the time plants begin to cup until early heading, if more than 20% of plants are infested, treatment is warranted. From early heading until harvest, the threshold again drops to 10% to protect market quality of the produce. Use pest-specific insecticides in early to mid-season when diamondback moths and cabbageworms are prevalent. Spinosans are another reduced-risk insecticide option. Spinosans are biologically based materials that are quite selectively active on caterpillar pests but are safe to beneficials. Many chemical insecticides are also effective in controlling caterpillar pests of cole crops.
Japanese beetles. (https://vegento.russell.wisc.edu/pests/japanese-beetle/)
Japanese beetles have only one generation per year. In late-June and in early July (or around 1000 growing degree days), adults begin emerging from the soil. Adult emergence has already begun across much of southern Wisconsin, but the bulk of the adult emergence is just beginning and will continue through early July.
Immediately thereafter, females mate with males and begin laying eggs. Adult beetles are most active in the afternoon in full-sun. Females leave ornamental plants where they feed and mate, and burrow two to four inches into the soil (under the turf and in mulched areas) in a suitable area to lay their eggs. Eggs hatch in about two weeks, after which grubs begin feeding on the roots of turfgrass and ornamental plants.
Mid-summer rainfall and adequate soil moisture are needed to prevent eggs and newly-hatched grubs from drying out. Adult females instinctively lay their eggs in areas with higher soil moisture content to ensure survival of their offspring. Older grubs are more drought-tolerant and will move deeper into the soil if conditions become dry. Grubs can also withstand elevated levels of soil moisture, so excessive rainfall or irrigation will not affect them.
As soil temperatures cool in the fall, and the first meaningful frost occurs, grubs begin to move deeper into the soil. Grubs overwinter in the soil about two to six inches below the surface, although some may be a deep as 20 inches. They become inactive when soil temperatures fall below 50°F. In the spring, when soil temperatures reach 50°F, the grubs begin to move up into the root-zone to resume feeding for about three to five weeks. Thereafter, the grubs stop feeding and begin creating an earthen cell where they pupate (i.e., transform) into adults.
Both the adults and grubs of Japanese beetles cause damage. Thus, controlling one life stage will not preclude potential problems with the other. Control options for each life stage are presented below.
Physical removal and trapping of adults: Removing beetles by hand, or trapping, may provide adequate protection for small plantings when beetle numbers are low. However, Japanese beetle adults can migrate from other areas, and the presence of beetles on or near a plant will attract more beetles. Consequently, use of Japanese beetle traps often attracts more beetles, and results in subsequent damage to plants.
Chemical control of adults: Several insecticides are labeled for use against adult Japanese beetles. Always follow label directions. Treat foliage and flowers thoroughly. For optimal control, apply in the afternoon when beetles are most active.
Cultural control of grubs: Because Japanese beetle eggs and young grubs have difficulty surviving in dry soil conditions, withholding irrigation during peak adult beetle flight may help to reduce grub populations. However, adequate soil moisture in late-August and September can help damaged turf recover from grub damage.
Biological control of grubs: Although there are a few biological control products that allegedly control Japanese beetle grubs, the performance of these products has been inconsistent. Biological control products include milky spore disease, insect-parasitic nematodes, and fungal pathogens such as Beauveria bassiana and Metarhizium.
Chemical control of grubs: Nearly all soil insecticides provide adequate control of Japanese beetle grubs. However, not all control products perform equally. The traditional approach has been to apply short- residual products after eggs have hatched, but before grubs cause visible damage. This approach is termed “curative” control. The optimal timing for curative treatments is early to mid-August. Carbaryl (Sevin), clothianidin (Arena) and trichlorfon are three active ingredients that all provide meaningful curative control. Preventative insecticides are another effective management option that is typically preferred over curative insecticides due to greater level of control and a larger application window of time, May to July, due to their longer residual activity. Preventative insecticides are best applied prior to egg lay, typically early July. Preventative products contain the active ingredients imidacloprid, chlorantraniliprole, clothianidin and thiamethoxam.
Asiatic garden beetle – (https://entomology.wisc.edu/2023/07/31/asiatic-garden-beetle-a-new-pest-to- have-on-your-radar/)
There is a new insect pest to have on your radar in Wisconsin—the Asiatic garden beetle (AGB) (Maladera formosae). This species can feed on and damage a wide range of plants including vegetable crops, field crops, fruit crops, turfgrass, and ornamental flowers, trees, and shrubs in nursery and landscape settings.

It can now be found across much of the eastern US. Over the last two decades, AGB has become more common in the Midwest with crop damage being reported in Indiana and southern Michigan in soybean, mint and potatoes. It is also established in parts of northern Illinois. Asiatic garden beetles were first collected in Wisconsin in July 2021 in a residential yard in Dane County (Middleton). Small numbers of adults continue to be spotted at that site, although no feeding or plant damage has been observed to date. In late July 2023, beetles were collected from a home garden in eastern Green Lake County; this is also the first confirmed report of plant damage in the state.
The AGB has a single generation each year, and adults have primarily been spotted in July in Wisconsin, although some specimens have been collected as late as September. Adults are nocturnal and feed almost exclusively after dark. If disturbed, they tend to tumble to the ground and hide. Adult AGBs are capable fliers and can come to lights in large numbers; blacklight traps can be a useful monitoring tool. In addition, adult flight activity is strongly associated with warm nighttime temperatures (70+˚ F). After mating, adult females lay eggs in soil. Reports from nearby states indicate that larvae may be more common in sandy soil compared to loamy areas.
Both the larvae and adults can feed on a wide range of plants. Adult AGBs tend to pack less of a punch than Japanese beetles but can chew irregular notches out of leaves. The larvae can be a considerable issue in potato fields when they normally feed on roots until vine-killing. After vine killing, roots will die, and the grubs will switch to other below-ground structures (tubers) to feed causing considerable damage. An abundance of AGB grubs resulting in below-ground wounds can serve as a potential entry point for pathogens that can cause storage rots.

Asiatic garden beetles belong to the same family as Japanese beetles and the larvae (white grubs) resemble this species. Larvae have pale, C-shaped bodies with three pairs of jointed legs and a brownish-orange head capsule and chewing mouthparts. They have a pale, bulbous structure at the base of their mouthparts which aids in diagnosis (no other white grubs in the Midwest have that feature). Adults AGBs are approximately 3/8 inch long, brownish, and resemble small May/June beetles; their elytra (wing covers) are also slightly iridescent.
Colorado potato beetle – (https://vegento.russell.wisc.edu/pests/colorado-potato-beetle/)
Overwintered adult Colorado potato beetle (CPB) are disappearing from southern and central Wisconsin potato fields. North of Hwy 10, adults are still prevalent and egg masses are still being laid throughout fields in northern Wisconsin. Later larval stages (3rd and 4th instar) are now common in central production fields. Northern production areas are seeing egg hatch and early larvae will be abundant in fields with the warm forecast temperatures.
CPB chemical management tools require different adjuvants and conditions for ideal performance. For example, most insecticides perform well under acidic tank mix conditions (pH 4-7), whereas active ingredients such as spinosad or spinetoram can perform well in slightly alkaline tanks (pH 7-8). Many insecticides can be described as ‘trans-laminar’, and this means the active ingredients need to gain access to the leaves on which they are applied. Shortly after application (eg. 15-30 minutes), these insecticides will penetrate the leaf cuticle (wax layer) and move in a very localized manner throughout the leaves on which they were applied (aka trans-laminar). In order to gain access to the interior of leaves, many of these insecticides require a mild penetrating surfactant. Consult the product listing provided with past newsletters to read about the specific conditions necessary to increase the efficacy of select active ingredients.
Amanda Gevens, Chair, Professor & Extension Vegetable Pathologist, UW-Madison, Dept. of Plant Pathology, 608-575-3029, gevens@wisc.edu, Lab Website: https://vegpath.plantpath.wisc.edu/.
Current P-Day (Early Blight) and Disease Severity Value (Late Blight) Accumulations will be posted at our website and available in the weekly newsletters. Thanks to Ben Bradford, UW- Madison Entomology for supporting this effort and providing a summary reference table: https://agweather.cals.wisc.edu/thermal-models/potato. A Potato Physiological Day or P-Day value of ≥300 indicates the threshold for early blight risk and triggers preventative fungicide application. A Disease Severity Value or DSV of ≥18 indicates the threshold for late blight risk and triggers preventative fungicide application. Data from the modeling source: https://agweather.cals.wisc.edu/vdifn are used to generate these risk values in the table below. I’ve estimated early, mid-, and late planting dates by region based on communications with stakeholders. These are intended to help in determining optimum times for preventative fungicide applications to limit early and late blight in Wisconsin.
| Planting Date | 50% Emergence Date | Disease Severity Values (DSVs)
through 6/28/2025 |
Potato Physiological Days (P-Days)
through 6/28/2025 |
||
| Spring Green | Early | Apr 5 | May 10 | 18 | 355 |
| Mid | Apr 18 | May 14 | 18 | 327 | |
| Late | May 12 | May 26 | 15 | 270 | |
| Arlington | Early | Apr 5 | May 10 | 14 | 354 |
| Mid | Apr 20 | May 15 | 14 | 317 | |
| Late | May 10 | May 24 | 11 | 281 | |
| Grand Marsh | Early | Apr 7 | May 11 | 14 | 338 |
| Mid | Apr 17 | May 14 | 14 | 319 | |
| Late | May 12 | May 27 | 14 | 265 | |
| Hancock | Early | Apr 10 | May 15 | 15 | 303 |
| Mid | Apr 22 | May 21 | 15 | 277 | |
| Late | May 14 | June 2 | 15 | 222 | |
| Plover | Early | Apr 14 | May 18 | 13 | 278 |
| Mid | Apr 24 | May 22 | 13 | 271 | |
| Late | May 19 | June 7 | 13 | 179 | |
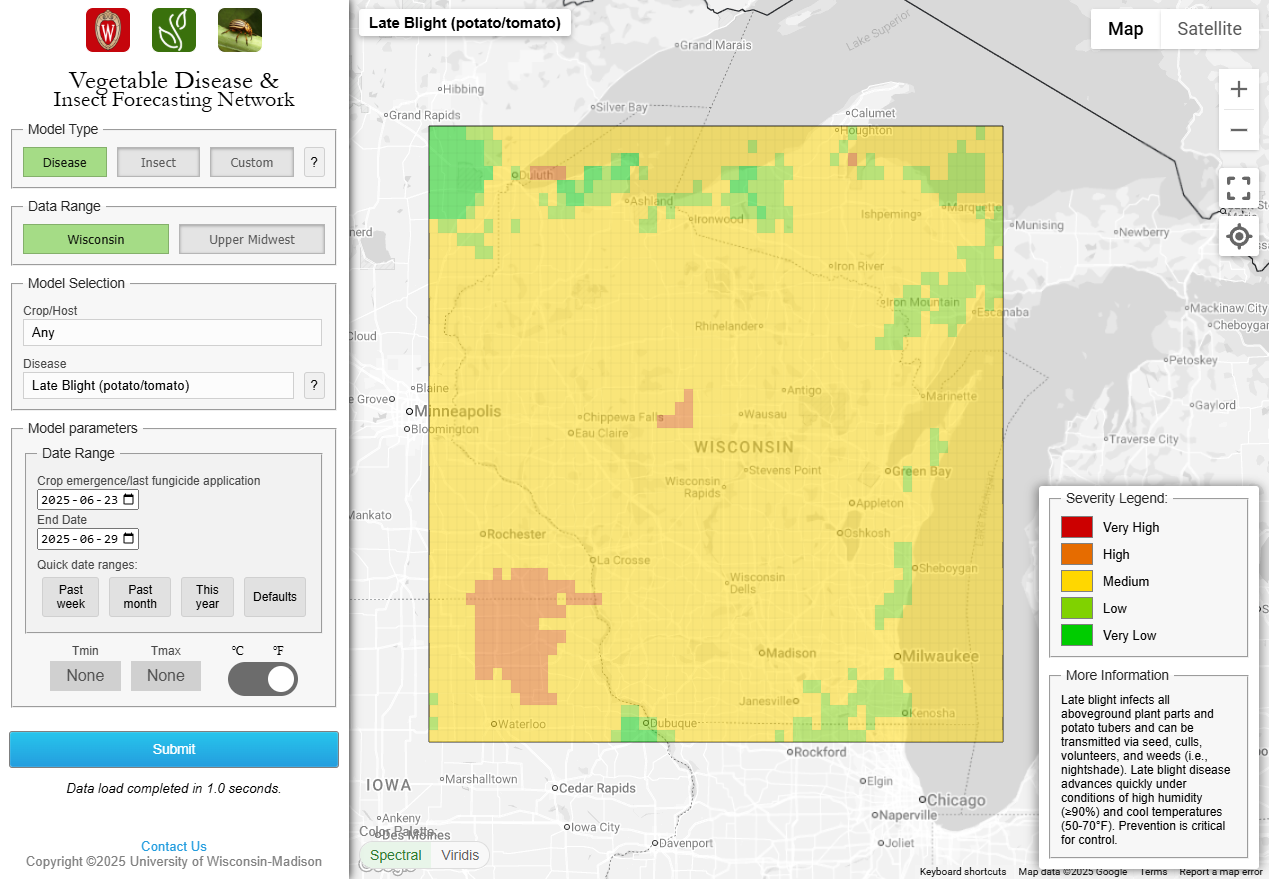
Late blight of potato/tomato. The usablight.org website indicates no new confirmed reports of late blight on tomato or potato in the US this past week. There was a US-23 late blight strain type confirmation in Collier County FL in 2025 (now > month old). The site is not comprehensive. This genotype/clonal lineage is generally still responsive to phenylamide fungicides meaning that Ridomil and Metastar fungicides (mefenoxam and metalaxyl) can still effectively control late blight caused by these strain types. We saw the accumulation of 2-12 DSVs across WI this past week, with the greatest accumulations in the southern part of the state. Early and mid-plantings of potatoes in the Spring Green area of WI have reached the Blitecast threshold of 18 DSVs and should receive preventative fungicides for the management of late blight. Please find a fungicide listing for Wisconsin potato late blight management at the following link: Potato Late Blight Fungicides.
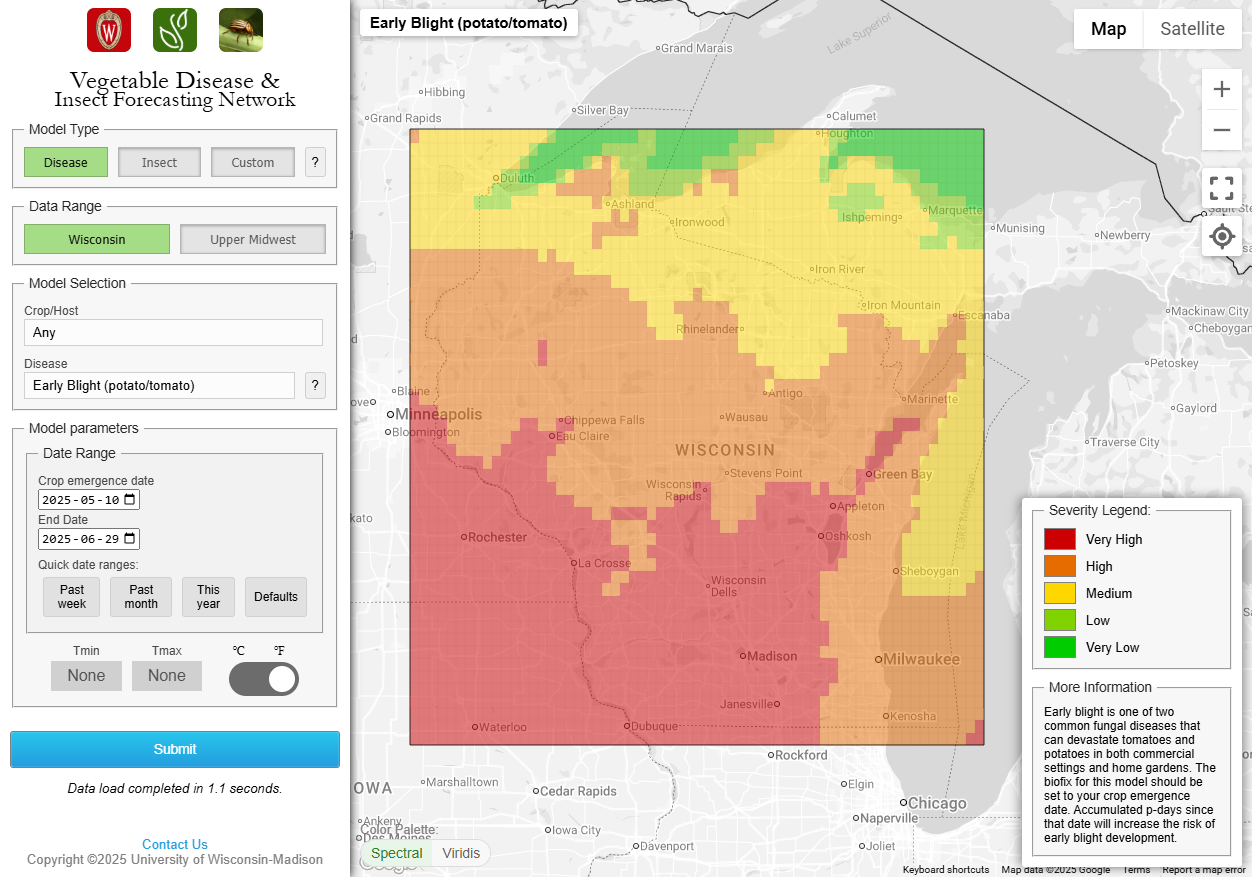
Early blight of potato. We continue to steadily increase P-Days in potatoes. Accumulations were roughly 55-58 over the past week with P-Day 300 thresholds met for preventative fungicide treatment in early planted potatoes in Spring Green, Arlington, Grand Marsh, and Hancock locations. Values will continue to amass and develop conditions optimum for early blight disease caused by Alternaria solani. Earliest inoculum typically comes from within a field and from nearby fields. Once established, early blight continues to create new infections due to its polycyclic nature – meaning spores create foliar infection and the resulting lesion on the plant can then produce new spores for ongoing new infections in the field and beyond. Early season management of early blight in potato can mitigate the disease for the rest of the season. Early blight lesions were seen in our UW Hancock ARS field trials this past week (photo below). Lower canopies of some varieties are yellowing creating a very favorable status for early blight infection. https://vegpath.plantpath.wisc.edu/diseases/potato-early-blight/

Fungicides can provide good control of early blight in vegetables when applied early on in infection. Multiple applications of fungicide are often necessary to sustain disease management to time of harvest due to the typically high abundance of inoculum and susceptibility of most common cultivars. For Wisconsin-specific fungicide information, refer to the Commercial Vegetable Production in Wisconsin (A3422), a guide available through the UW Extension Learning Store website which is annually updated.
For custom values, please explore the UW Vegetable Disease and Insect Forecasting Network tool for P- Days and DSVs across the state (https://agweather.cals.wisc.edu/vdifn). This tool utilizes NOAA weather data. Be sure to enter your model selections and parameters, then hit the blue submit button at the bottom of the parameter boxes. Once thresholds are met for risk of early blight and/or late blight, fungicides are recommended for optimum disease control. Fungicide details can be found in the 2025 Commercial Veg. Production in WI Extension Document A3422: https://cropsandsoils.extension.wisc.edu/articles/2025-commercial-vegetable-production-in-wisconsin- a3422/
Cucurbit Downy Mildew: This national cucurbit downy mildew information helps us understand the potential timing of arrival of the pathogen, Pseudoperonospora cubensis, in our region, as well as the strain type which can give us information about likely cucurbit hosts in WI – as well as best management strategies. Clade 1 downy mildew strains infect watermelon and Clade 2 strains infect cucumber. I am hosting a cucurbit (and basil) downy mildew sentinel plot at the UW Hancock Agricultural Research Station this summer. This ‘sentinel plot’ is a non-fungicide-treated collection of cucurbit plants which are observed weekly for disease symptoms. No downy mildew was seen on basil or cucurbits this past week. Additionally, I keep an eye on the downy mildew work of Dr. Mary Hausbeck at Michigan State University and include this information as relevant to WI https://veggies.msu.edu/downy-mildew-news/. This season, Clade 2 downy mildew spores were confirmed in multiple MI counties and downy mildew has been confirmed in commercial cucumber fields in 4 southern MI counties (reported 6/26/25). The outbreak is in pickling cucumber crops in southeast Michigan (Monroe and Lenawee counties) and southwest Michigan (Cass and VanBuren counties) and has unfolded in just a few days. More information: https://www.canr.msu.edu/news/downy-mildew-confirmed-on-cucumbers-in-four-michigan-counties2025
Treatment recommendations from Michigan State University include:
- Elumin + chlorothalonil or mancozeb
- Omega (Orbus) + chlorothalonil or mancozeb
- *Orondis Opti (chlorothalonil is part of the premix, additional chlorothalonil is suggested; see label for maximum chlorothalonil rates)
- Previcur Flex + chlorothalonil or mancozeb
- *Ranman + chlorothalonil or mancozeb
- Zampro + chlorothalonil or mancozeb
*Products considered to be especially effective in Michigan based on yearly, season-long field trials.
Potato Blackleg and Aerial Stem Rot – blackleg on potato caused by pectolytic bacteria, primarily Pectobacterium spp. has been abundant in fields in Wisconsin over the past week or so. Samples processed through our UW Plant Disease Diagnostic Clinic have resulted in findings of Pectobacterium carotovorum subsp. carotovorum and Pectobacterium parmentieri primarily. Other pathogens that can be associated with these symptoms can include Pectobacterium atrosepticum and Dickeya spp. (The content below includes some elements from Dr. Amy Charkowski of Colorado State Univ., Dr. Phillip Wharton of Univ. of Idaho & a Blackleg, aerial stem rot, and tuber soft rot fact sheet from Michigan State Univ.).

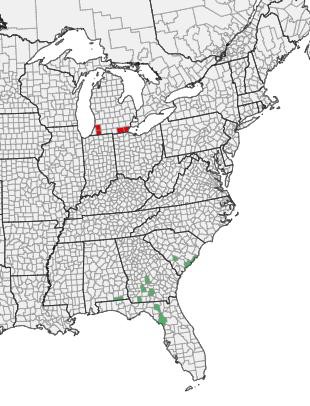
Disease Background: The primary bacterial pathogens that cause aerial stem rot, potato blackleg and tuber soft rot are Pectobacterium atrosepticum, P. carotovorum, P. wasabiae, P. parmentieri, and more recently in the U.S., Dickeya spp. Previously, all of these pathogens were grouped in the same genus Erwinia. Dickeya and Pectobacterium affect many host species including potato, carrot, broccoli, corn, sunflower and parsnip; legumes and small grains are not known hosts. Dickeya dianthicola was confirmed in the eastern U.S. in just 2015, causing significant potato losses in some areas. Dickeya appears to spread over long distances via seed potatoes, was first reported in the Netherlands in the 1970s, and has since been detected in many other European countries, and now the U.S. This summer, so far in Wisconsin, has yielded blackleg and aerial stem rot primarily caused by Pectobacterium spp.
Under the right environmental conditions, infection of seed with blackleg pathogens can result in symptoms including poor emergence, chlorosis, wilting, leaf curling, tuber and stem rot, and darkened or black stems which are slimy, and death. These symptoms result from the cell-wall-degrading enzyme activity of the bacteria within the plant tissues on which they infect.
Aerial stem rot, blackleg and soft rot bacterial diseases are typically promoted by cool, wet conditions at planting and high temperatures after emergence. While the pathogens can be spread in infested seed, other sources of inoculum include soil, irrigation water, and insects. Levels of infection are dependent upon seed-handling/cutting techniques, soil moisture and temperature at planting and emergence, cultivar susceptibility, severity of infection of seed, and potentially, amount of bacteria in irrigation water, cull piles, or other external sources. Sanitation and disinfesting of potato cutting equipment and proper handling reduces spread and aids in control of the pathogen.
Treating seed to prevent seed piece decay by fungi can also contribute to blackleg control. Since the pathogen does well in cool, wet soils, avoid planting in overly wet soil. Crop rotation away from potato for 2-3 years for Pectobacterium and less than 1-2 years for Dickeya species will help control this disease as the bacteria do not survive well in soil.
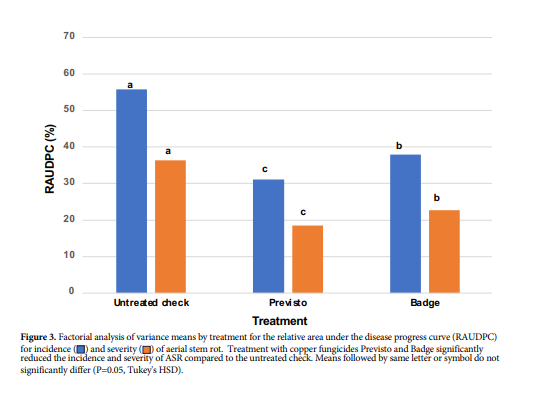
While seedborne or vascular blackleg (internal inoculum) cannot be reversed with applications of fungicides or bacteriacides, spread of the bacterial pathogen from infected to healthy plants and aerial stem rot (following damage to plants) may be managed in the field with fungicide tank-mixes that contain copper. In 10 years of field trials in Idaho (under the direction of Dr. Phillip Wharton of Univ. of Idaho) evaluating weekly applications of copper-containing fungicides, aerial stem rot was most effectively controlled with Previsto (reduced disease incidence and severity by 25 and 18% respectively). Previsto is a Gowan product with 5% copper hydroxide.
Badge also significantly reduced aerial stem rot when applied weekly (reduced disease incidence and severity by 18 and 14% respectively). Badge is also a Gowan product with 16.81% copper oxychloride and 15.36% copper hydroxide. Most often, conditions that favor plant-to-plant spread include high winds and driving rains or heavy overhead irrigation. Cultivars vary in susceptibility and some can have a tendency for lower canopy leaf scarring providing infection sites for stem rotting bacteria.
In work by Dr. Dennis Johnson of Washington State University, the famoxadone+cymoxanil (Tanos) plus mancozeb tank-mix alternated with mancozeb+copper hydroxide (ie: Kocide) was an effective chemical tool in reducing aerial stem rot in potato. Irrigation management to reduce excess water also greatly enhanced control of aerial stem rot. Copper hydroxide applications alone did not have as effective of control as Tanos+copper hydroxide. As Tanos is also an excellent late blight control material, its use as we surpass DSVs of 18 at this time offers an appropriate program for control of both diseases.
Although disease symptoms are often indistinguishable from those of the more established blackleg pathogen Pectobacterium spp., Dickeya spp. can initiate disease from lower inoculum levels, have a greater ability to spread through the plant’s vascular tissue, are considerably more aggressive, and have higher optimal temperatures for disease development. Dickeya is not a good soil survivor (generally <2 years) and rotation out of potato for at least 3 years will greatly reduce the disease. Dickeya and Pectobacterium thrive in water and low oxygen, and therefore over-irrigation, poor drainage or excessive rain will spread Dickeya and Pectobacterium. Both pathogens can spread after severe storms.
Generally, disease caused by Dickeya spp under warm, wet conditions leads to stem rotting with symptoms similar to those of P. atrosepticum. Under conditions with lower humidity, less rotting is observed with Dickeya spp but symptoms such as wilting, increased leaf desiccation, stem browning and hollowing of the stem can be present. Tuber soft rot, from either pathogen, ranges from a slight vascular discoloration to complete decay.
Affected tuber tissue is cream to tan and is soft and granular. Brown to black pigments often develop at the margins of decayed tissue. Lesions usually first develop in lenticels, at the site of stolon attachment or in wounds. Symptoms caused by Dickeya spp. tend to develop when temperatures exceed 25ºC (77ºF), while Pectobacterium predominate below 25ºC.Recent studies showed that Dickeya spp., particularly at temperatures of 27ºC (80ºF) or above, cause more severe rots than P. atrosepticum and are more likely to produce a creamier, cheesy rot.
Dickeya dianthicola, the relatively newer blackleg pathogen in the US, has the ability to remain dormant in tubers when temperatures are low (for example, at harvest time and in seed storages). Tubers infected with this form of Dickeya look healthy at planting, but the disease develops when soil temperature increases. Seed tubers may rot in the soil, causing poor emergence, or infected plants may emerge that eventually die but not before spreading the disease to neighboring plants. We have been detecting far less Dickeya spp. from symptomatic plants and tubers over the past few years in Wisconsin. This has also been a national trend.
Cutting seed will spread Pectobacterium within a seed lot. After several years of specific testing, Dickeya has been found to NOT spread by standard cutting approaches. If cutting seed, it’s important to ensure that the cut surfaces are suberized prior to planting to avoid new infections. Dickeya may be managed through biosecurity measures and on-farm precautions such as decontamination of farm machinery, eliminating plant debris and alternative hosts, and avoidance of mechanical harvesting during the early phases of pre-basic seed tuber multiplication.
Growers should make sure to thoroughly sanitize seed cutting equipment and planter between seed lots to mitigate pathogen spread (for several other pathogens). Seed should be warmed prior to planting so that it is approximately the same temperature as the soil, and to reduce water condensation on tubers. Bacteria cannot enter plant tissues unless there is a port of entry (for example, un-suberized cut surfaces of the seed tuber, or bruises) and a film of water or a wet surface. At harvest, growers should reduce the chances of inflicting damage to the skin such as cuts and bruises. If soft rot is present in a portion of the field, this part of the field should not be harvested. In addition, harvesting equipment should be sanitized between lots. Improved storage management can reduce bacterial load on tubers and tuber rotting. Both physical (especially hot water treatment) and chemical methods have been explored with limited success.
Dickeya grows slowly or not at all at seed storage temperatures, so if the crop looks good going into storage, it will likely not decay in storage due to Dickeya, but the bacteria will likely cause disease and spread the next year if infected potatoes are planted.
Testing for Dickeya and Pectobacterium is available using new standard polymerase chain reaction (PCR) assays. Our UW Plant Disease Diagnostic Clinic can provide these tests.
Grower Checklist for Managing Pectobacterium & Dickeya
- Plant certified, disease-free tubers, into well-drained soil with temperature under 10°C.
- Plant whole seed tubers if Suberize cut seed before planting.
- Plant seed tubers during conditions that favor fast
- Clean and disinfest tools and equipment used for cutting and planting
- Avoid wounding during seed cutting, planting and
- Fungicidal seed treatment of potatoes to prevent seed piece decay can indirectly prevent seed contamination, especially during the cutting operation.
- Utilize crop rotation of two or more years with a non-host
- Avoid over-
- Avoid excessive fertilization, which may impact plant and tuber
- Consider copper fungicides, which are partially effective against disease and dry out existing
- Delay harvest until skin set is complete (up to 21 days after top-kill).
- Avoid wet conditions during harvest to prevent soil from sticking to tuber
- Store contaminated potato lots
- Provide adequate ventilation in
- Check storages regularly for temperature increase and If problems are detected, hot-spot fans can be used to cool the pile.
- Dry potatoes before storage or shipping.


