
In This Issue:
- Impacts of current weather on potato productivity
- Water quality program – irrigation water testing for nitrogen
- Disease forecasting updates for potato early blight and late blight
- Cucurbit downy mildew updates
- Squash vine borer, Potato leafhopper, Cucumber beetles, Aster leafhopper, Colorado Potato beetles and their management
Yi Wang, Associate Professor & Extension Potato and Vegetable Production Specialist, UW-Madison, Dept. of Plant and Agroecosystem Sciences, 608-265-4781, Email: wang52@wisc.edu.
Based on the weather data from the Hancock weather station shown on the Wisconet webpage, the past two weeks had multiple rainy days with high temperatures (above 80°F). Late June climate probabilities are showing a trend toward above-normal temperatures and precipitation. A risk of extreme heat over the next few days makes efficient irrigation scheduling critical. For July, the climate outlook indicates a potential warmer-than-normal month with precipitation uncertainty.
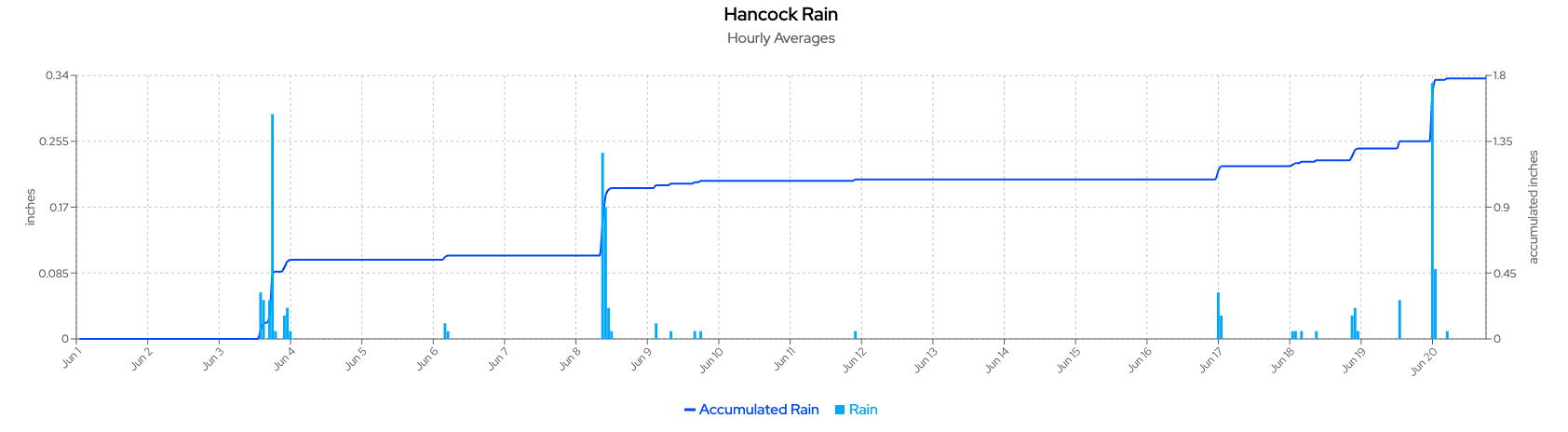
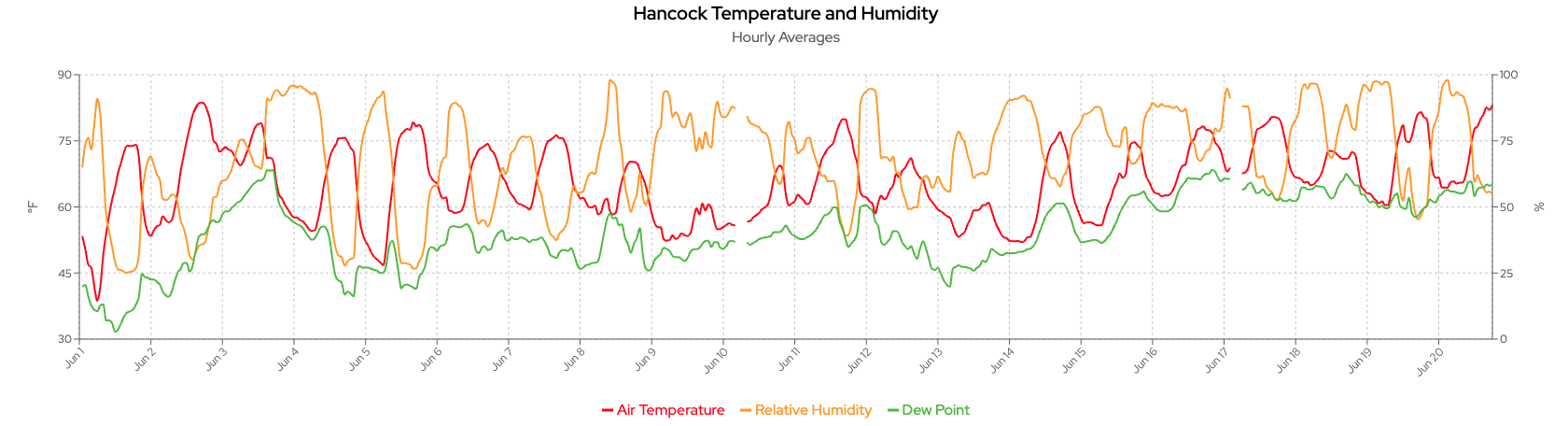
Since most potatoes planted in April have reached canopy closure and are vigorously bulking tubers, managing soil moisture to fluctuate between 80% and 100% field capacity will be important to avoid any water deficiency issues. Some growers started to see heat sprouts (runners) on Russet Burbanks (Figures below) recently.

Heat sprouts (also called heat runners) are a physiological defect caused by high soil temperatures (with or without soil moisture deficiency). Under heat stress, tubers sprout prematurely because the heat breaks their dormancy. The heat sprout will either grow into a leafy aboveground stem or another tuber (a chain tuber). This physiological issue will cause reduced yield and quality as well as reduced storage life. In general, heat stress during the field season can shorten the period of dormancy in potato tubers, making them more likely to sprout in storage even under optimal storage conditions.
Heat stress can also cause secondary growth or knobbiness on potato tubers (Figure on the right). Under heat stress, normal cell division and tuber expansion are interrupted and paused. When better growing conditions return, cell division and tuber growth will resume, but in a different direction, leading to malformed tubers that are not marketable.

Some varieties, such as Russet Burbank, are more susceptible to heat stress than others. The upcoming heat stress may cause more heat runners or knobbiness on Burbanks.
Some tips to manage heat stress: Irrigation scheduling: Provide adequate and timely irrigation to cool the soil and reduce the impact of heat stress. Never allow soil moisture to drop below 65% of field capacity on the sands at this growth stage.
Nutrient management: Calcium is a key nutrient that can help potato plants reduce heat stress and improve tuber quality. Calcium nitrate can help mitigate the adverse effects of heat stress by reducing the damage caused by reactive oxygen species within the plants. Sufficient calcium can also help maintain normal cell functions under high soil temperatures, including the opening and closing of stomata and cell division. Meanwhile, nitrogen management is also crucial to ensure the general health of the plants.
Cultural practice: Maintain uniform planting depths and ensure proper soil drainage (linked to good soil health).
Guolong Liang, Outreach Specialist, University of Wisconsin Madison Division of Extension, E-mail: gliang6@wisc.edu, Phone: 715-540-8653
The UW–Madison Extension Agriculture Water Quality Program is excited to announce a new program that offers free irrigation water testing and custom nitrogen assessments.
Nitrogen in irrigation water is often overlooked in nutrient management—but it can make a meaningful difference in your bottom line and water quality. This project helps you make more precise nitrogen decisions for the future.
Who should join? Farmers using high-capacity irrigation wells.
What we offer: 1) Free water testing: Nitrate-N content in irrigation water. 2) Custom report: A detailed nitrogen balance tailored for your fields. 3) 1-on-1 support: Help interpret data and assess potential to increase efficiency.
How does it benefit you? 1) Gain awareness of nitrogen content in irrigation water. 2) Assess potential to save money on inputs and improve water quality. 3) Make more precise nitrogen decisions for the future.
Interested in participating? Fill out this intake form and an outreach specialist will reach out to you. Apply by July 15, 2025. If you have any questions, contact Guolong Liang, Outreach Specialist, at gliang6@wisc.edu or (715) 540-8653.
Amanda Gevens, Chair, Professor & Extension Vegetable Pathologist, UW-Madison, Dept. of Plant Pathology, 608-575-3029, gevens@wisc.edu, Lab Website: https://vegpath.plantpath.wisc.edu/.
Current P-Day (Early Blight) and Disease Severity Value (Late Blight) Accumulations will be posted at our website and available in the weekly newsletters. Thanks to Ben Bradford, UW-Madison Entomology for supporting this effort and providing a summary reference table: https://agweather.cals.wisc.edu/thermal-models/potato. A Potato Physiological Day or P-Day value of ≥300 indicates the threshold for early blight risk and triggers preventative fungicide application. A Disease Severity Value or DSV of ≥18 indicates the threshold for late blight risk and triggers preventative fungicide application. Data from the modeling source: https://agweather.cals.wisc.edu/vdifn are used to generate these risk values in the table below. I’ve estimated early, mid-, and late planting dates by region based on communications with stakeholders. These are intended to help in determining optimum times for preventative fungicide applications to limit early and late blight in Wisconsin.
|
|
Planting Date | 50% Emergence Date | Disease Severity Values (DSVs)
through 6/21/2025 |
Potato Physiological Days (P-Days)
through 6/21/2025 |
|
| Spring Green | Early | Apr 5 | May 10 | 9 | 300 |
| Mid | Apr 18 | May 14 | 9 | 272 | |
| Late | May 12 | May 26 | 6 | 215 | |
| Arlington | Early | Apr 5 | May 10 | 6 | 297 |
| Mid | Apr 20 | May 15 | 6 | 260 | |
| Late | May 10 | May 24 | 3 | 223 | |
| Grand Marsh | Early | Apr 7 | May 11 | 3 | 279 |
| Mid | Apr 17 | May 14 | 3 | 260 | |
| Late | May 12 | May 27 | 3 | 205 | |
| Hancock | Early | Apr 10 | May 15 | 3 | 246 |
| Mid | Apr 22 | May 21 | 3 | 219 | |
| Late | May 14 | June 2 | 3 | 165 | |
| Plover | Early | Apr 14 | May 18 | 1 | 221 |
| Mid | Apr 24 | May 22 | 1 | 214 | |
| Late | May 19 | June 7 | 1 | 122 | |
| Antigo | Early | May 1 | May 24 | 6 | 184 |
| Mid | May 15 | June 1 | 6 | 149 | |
| Late | June 1 | June 15 | 1 | 59 | |
| Rhinelander | Early | May 7 | May 25 | 5 | 169 |
| Mid | May 18 | June 8 | 5 | 98 | |
| Late | June 2 | June 16 | 1 | 52 | |
Late blight of potato/tomato. The usablight.org website indicates no new confirmed reports of late blight on tomato or potato in the US this past week. There was a US-23 late blight strain type confirmation in Collier County FL in 2025 (now > month old). The site is not comprehensive. This genotype/clonal lineage is generally still responsive to phenylamide fungicides meaning that Ridomil and Metastar fungicides (mefenoxam and metalaxyl) can still effectively control late blight caused by these strain types. We saw the accumulation of 0-6 DSVs across WI this past week, with the greatest accumulations in the southern part of the state.
Early blight of potato. We continue to steadily increase P-Days in potatoes. Accumulations were roughly 60 over the past week. Values will continue to amass and develop conditions optimum for early blight disease caused by Alternaria solani. Earliest inoculum typically comes from within a field and from nearby fields. Once established, early blight continues to create new infections due to its polycyclic nature – meaning spores create foliar infection and the resulting lesion on the plant can then produce new spores for ongoing new infections in the field and beyond. Early season management of early blight in potato can mitigate the disease for the rest of the season. No early blight was seen in our UW Hancock ARS field trials this past week. https://vegpath.plantpath.wisc.edu/diseases/potato-early-blight/
Fungicides can provide good control of early blight in vegetables when applied early on in infection. Multiple applications of fungicide are often necessary to sustain disease management to time of harvest due to the typically high abundance of inoculum and susceptibility of most common cultivars. For Wisconsin-specific fungicide information, refer to the Commercial Vegetable Production in Wisconsin (A3422), a guide available through the UW Extension Learning Store website which is annually updated.
For custom values, please explore the UW Vegetable Disease and Insect Forecasting Network tool for P-Days and DSVs across the state (https://agweather.cals.wisc.edu/vdifn). This tool utilizes NOAA weather data. Be sure to enter your model selections and parameters, then hit the blue submit button at the bottom of the parameter boxes. Once thresholds are met for risk of early blight and/or late blight, fungicides are recommended for optimum disease control. Fungicide details can be found in the 2025 Commercial Veg. Production in WI Extension Document A3422: https://cropsandsoils.extension.wisc.edu/articles/2025-commercial-vegetable-production-in-wisconsin-a3422/
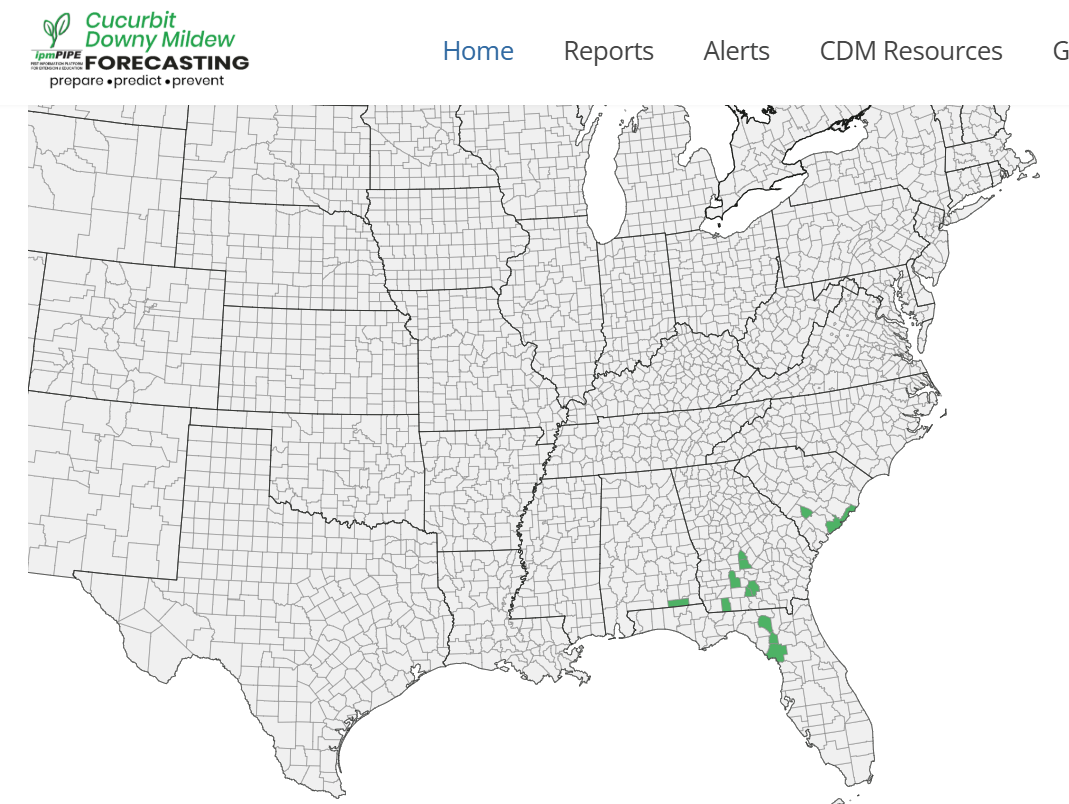
Cucurbit Downy Mildew: This national cucurbit downy mildew information helps us understand the potential timing of arrival of the pathogen, Pseudoperonospora cubensis, in our region, as well as the strain type which can give us information about likely cucurbit hosts in WI – as well as best management strategies. Clade 1 downy mildew strains infect watermelon and Clade 2 strains infect cucumber. I am hosting a cucurbit (and basil) downy mildew sentinel plot at the UW Hancock Agricultural Research Station this summer. This ‘sentinel plot’ is a non-fungicide-treated collection of cucurbit plants which are observed weekly for disease symptoms. No downy mildew was seen on basil or cucurbits this past week. Additionally, I keep an eye on the downy mildew spore trapping work of Dr. Mary Hausbeck at Michigan State University and include this information as relevant to WI https://veggies.msu.edu/downy-mildew-news/. This season, Clade 2 downy mildew spores were confirmed in 5 MI counties. Cucurbit Downy Mildew – UW Vegetable Pathology – UW–Madison

Vegetable Insect Update – Russell L. Groves, Professor and Department Chairperson, UW-Madison, Department of Entomology, 608-262-3229 (office), (608) 698-2434 (cell), e-mail: rgroves@wisc.edu
Vegetable Entomology Webpage: https://vegento.russell.wisc.edu/
Squash vine borer – (https://vegento.russell.wisc.edu/pests/ssedcorn-maggot/)
Beginning in late June to early July, adult vine borers emerge from the ground. In the Midwest, the pest typically emerges after 800-900 growing degree-days (base 50°C) have been reached. Often, this degree day threshold will closely coincide with full bloom of the common roadside weed chicory (Chicorium intybus L.). The risk for egg laying is illustrated below in the image from the Wisconsin Vegetable Disease and Insect Forecasting site. According to the model illustrated below, the adult moths will emerge soon and will soon mate and begin laying eggs thereafter.

Newly emerged female moths quickly seek suitable hosts and begin laying small, brown eggs singly at the base of susceptible plants. Each female is capable of laying between 150-200 eggs. Depending upon temperature, eggs will hatch within 10-15 days of being laid. Newly hatched larvae quickly bore into the vine stems to feed for four to six weeks. As the larvae feed, they leave behind characteristic light brown frass (insect feces) that resemble sawdust. Larvae typically feed at the center of host plant stems. This type of internal feeding (girdling) greatly restricts the plant’s ability to move water and nutrients, and the plant can look wilted at the time of larval infestation. The damage caused by squash vine borer larvae is often difficult to detect until the plant wilts and dies in late July and August. Initial signs of infestation are very difficult to detect. Scouting early often involves searching for entrance holes and frass at the base of vine crop stems.
Advanced symptoms of squash vine borer infestation are quickly wilting plants in the heat of the day. Since wilting may be confused with other pests of vine crops (e.g., bacterial or Fusarium wilts) scouting remains critical. Plants that are infested may be diagnosed by splitting the base stems of the plant to confirm the presence of the larvae. Fields that have been damaged in past seasons have a good chance for recurring squash vine borer infestations annually. Fully-grown borers exit the stems and burrow into the soil to pupate. Squash vine borers produce only one generation per year in Wisconsin.
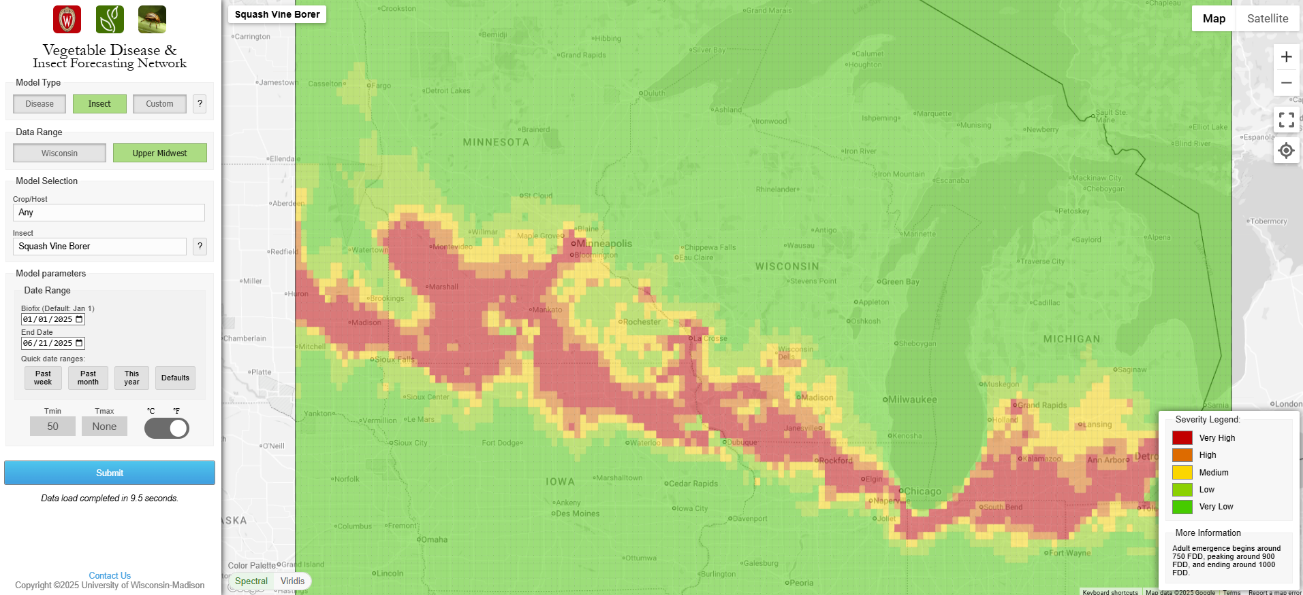
In Wisconsin, infestation risk can be reduced by planting crops early in the season. Floating row cover placed on the crop when adults are actively laying eggs is an effective method to reduce problems. Understanding when vine borers are present is a critical component to successful management with floating row cover. Synchronizing row cover installation with peak adult activity will reduce damage to preferred host plants. Keep in mind that plants in bloom require bees to pollinate the flowers. Planting a trap crop such as summer squash can be an effective means of reducing damage in the primary crop. Trap crops should be planted early to provide a more attractive egg deposition area than less preferred cucurbit species. Trap crop residue should be destroyed before larvae exit vines to pupate, limiting next season’s infestation.
Squash vine borer is very difficult to manage with chemical insecticides since older larvae are protected within the plant stem. The target life-stage for conventional chemical management is newly hatched larvae that have not yet entered the stem. Effective control requires insecticide residue to be in place before and during the egg laying period (950-1,000 DD50). Two to three successive applications of insecticide 5-7 days apart will adequately control most of the larval borers before entering the vines. Foliar-applied materials containing spinosad, Bacillus thuringiensis subsp. kurstaki, and chlorantraniliprole are effective and reduced risk options.
Potato leafhopper – (https://vegento.russell.wisc.edu/pests/potato-leafhopper/)
Populations of adult potato leafhopper (PLH) are now increasing in many parts of southern Wisconsin, especially in areas adjacent to alfalfa cut in late May. Recall, these are annual pests of snap beans, hops, clover, alfalfa and potatoes. Both adults and nymphs feed by inserting their mouth parts into the plant’s phloem and extracting sap and thereby injecting saliva containing toxic substances. It is the plant’s response to the saliva (salivary proteins) that results in the observed damage.
Migratory populations of the PLH entered the southern portion of Wisconsin in late May, and populations continue to develop in forage crops. For this reason, regular scouting of beans, potatoes, hops and alfalfa needs to be in place now to ensure nymphal populations are not building in number. Leafhopper populations can build over successive weeks before any damage symptoms begin to show, and it is critical to gain control before they display the “hopperburn” symptomology. Leafhoppers tend to move into other crops in early summer and as second cutting of forage crops is underway in many locations of the state, it is a key time to scout for early migrants in vegetable plantings.
Snap beans and potatoes should be scouted regularly for PLH activity. Leafhoppers tend to migrate into other crops in early summer after alfalfa is cut. This is a key time to watch for early migrants in vegetable plantings. With snap beans, the greatest amount of injury caused by PLH occurs during the seedling stage.

Commercial vegetable growers should use sweep nets and sticky cards at field edges to monitor adult populations in their fields. Take 25 sweeps with an insect sweep net per sample site. Use at least 5 sample sites per 30 acres. Nymph populations should be monitored by visual examination of the undersides of 25 leaves per sample site. Select leaves from the middle to lower half of the plant.
Cucumber beetles. – (https://vegento.russell.wisc.edu/pests/cucumber-beetles/)
Striped and spotted cucumber beetles will now begin to infest many of our cucurbit crops planted over the past 3 weeks. Spotted cucumber beetle (aka southern corn rootworm) can cause damage in vine crops, but the striped beetle is more common and damaging in Wisconsin. Feeding from larvae and adults causes direct damage to roots, leaves, flowers, and fruits.
 Adult striped cucumber beetle can vector the bacteria, Erwinia tracheiphila. Cucumbers and melons are particularly susceptible to bacterial wilt, and damage from this can be severe. Only the striped cucumber beetle overwinters in Wisconsin. They emerge in mid- to late May and lay eggs in the soil at the base of cucurbits. Spotted cucumber beetles migrate to northern locations in early to mid-July. This late arrival seldom makes them a serious problem.
Adult striped cucumber beetle can vector the bacteria, Erwinia tracheiphila. Cucumbers and melons are particularly susceptible to bacterial wilt, and damage from this can be severe. Only the striped cucumber beetle overwinters in Wisconsin. They emerge in mid- to late May and lay eggs in the soil at the base of cucurbits. Spotted cucumber beetles migrate to northern locations in early to mid-July. This late arrival seldom makes them a serious problem.
Plants infected with bacterial wilt will not recover. Therefore, it is important to control beetles early in the season to prevent the spread of the disease. Scout fields for adult beetles 2-3 times per week early in the season and weekly thereafter. Particular attention is needed in field edges where beetles congregate. The treatment threshold for cucumber beetles is 1 beetle per plant in melons, cucumber, Hubbard and Butternut squash, and younger pumpkins and 5 adults per plant in watermelon, other varieties of squash and older pumpkins. Beetle populations in excess of 20 per plant may transmit the bacterial wilt before insecticides have a chance to control the beetles.
Non-chemical control is possible in small plantings by covering the plants with floating row covers. Be sure to uncover flowering plants to allow bees to enter and pollinate the plants. Rotating crops with grain, tomatoes, or a cover crop or using perimeter trap crops can delay infestations. If a trap crop is used, exercise care that the trap crop will not function as a reservoir for bacterial wilt. If bacterial wilt infections have already occurred, remove the diseased plants immediately to prevent the spread of the disease while insects are present.
Aster leafhopper (https://vegento.russell.wisc.edu/pests/aster-leafhopper/)
The aster leafhopper is a serious pest of many crops in the upper Midwest because of its ability to spread aster yellows disease. Aster yellows is untreatable and the only solution is to remove infected plants and treat for the leafhopper if numbers are too great. Both the insect and the disease can attack a broad range of plants, including vegetables, field crops, flowers, and weeds. Infected flowers, particularly those in the aster family (Compositae), are severely disfigured by the disease, destroying both their visual appeal and their economic value.

Leafhoppers prefer lettuce, carrots, celery, and small grains for feeding and breeding, while other crops such as potatoes and onions provide a temporary source of food and refuge. Only adults use these temporary sites; immature leafhoppers fail to develop on these plants. All plants are susceptible to aster yellows infection.
Migratory populations of the aster leafhopper have arrived in Wisconsin over the past 5-7 weeks and, similar to other migratory pests mentioned (e.g., cutworm, potato leafhopper) are associated with the recent storm events that have brought southern winds into the state in advance of the fronts. Adult leafhoppers that migrate into the state can be infected with the bacterium that causes the disease.
Begin scouting for aster leafhoppers in early spring when plants are newly sprouted and continue scouting weekly throughout the end of July. Yellow sticky cards may be placed in the field to determine when the first migrants arrive. Once leafhoppers are observed, you will need to estimate the size of the population using an insect net to sweep the area. Take 100 sweeps per sample site and sample at least four areas. Established thresholds for treatment vary by crop susceptibility but are generally: i) susceptible carrot varieties: 20 adult ALH/100 sweeps; ii) resistant carrot varieties: 40 adult ALH/100 sweeps; iii) onion, 15 adult ALH/100 sweeps; iv) celery: 10 adult ALH/100 sweeps. Consult A3422 (Commercial Vegetable Production in Wisconsin), to get a listing of carrot varieties considered susceptible and resistant.
Colorado potato beetle – (https://vegento.russell.wisc.edu/pests/colorado-potato-beetle/)
Continue to scout populations of Colorado potato beetle (CPB), especially as nearly all potato plants have emerged throughout much of Wisconsin. In southern Wisconsin, adult colonization is nearly complete in most fields and less than 25% of egg masses remain unhatched. Later larval stages (3rd and 4th instar) are now common in southern locations, whereas mostly early instar larvae are present in portions of the state north of Hwy 21. In areas of the state north of Rosholt, WI adults are just beginning to lay eggs, and the first eggs will be hatching soon. In each instance, the choice of insect control products can vary widely. Northern production areas can still use perimeter treatments (e.g., indoxacarb) and insect growth regulators (e.g., novaluron), whereas central and southern locations will not benefit as much from these treatments at this time.
Recall, there can be considerable variability in the predominant lifestages present, and this often results from planting date (later dates have younger larvae) and proximity to previous year potato (larger larvae in fields close to previous year potato).

For most CPB chemical management tools, timing of applications occur with the appearance of first instar larvae in the field is critical. Early instar larvae are the most susceptible life stage(s) for chemical management, and applications should be timed with the midpoint of egg hatch. The first application should be followed up in 7-10 days later with a second application of the same compound depending on the formulation and label restrictions. Refer to the UW-Extension publication Commercial Vegetable Production in Wisconsin (A3422) for a list of registered insecticides and management recommendations. We have summarized chemical options for CPB management on our website: https://vegento.russell.wisc.edu/extension/colorado-potato-beetle-management/.
Applications of ledprona (Calantha), novaluron (Rimon) tolfenpyrad (Torac), spinetoram (Radiant, Delegate), or abamectin (Agri-Mek) should be applied when 50-75% of egg masses have hatched, and a few 2nd instar larvae are present from the earliest hatched egg masses. This milestone was reached last week in many fields in central Wisconsin, with several egg masses continuing to be being deposited as overwintered adults continue to be active in many fields in the Central Sands. These 1st generation larvicides often require 2-3 subsequent re-applications spaced on a 7-10 day interval to achieve sufficient control.
In northern Wisconsin, CPB adults are still colonizing fields, and mating and egg laying are just underway along field perimeters. With warm day and nighttime temperatures forecast for the coming week, populations will move fast so do not delay! Careful scouting will reveal the exact timing! Recommended products for control are provided in the attached list of products. Also carefully note the appropriate timing of application and associated adjuvants that are needed to enhance the control of the specific active ingredient.

